|
|
||||
|
Interpreting
Electrospray Mass Spectra |
||||
| -- | ||||
| Calculating Mass | ||||
| -- | ||||
|
FAQ |
||||
|
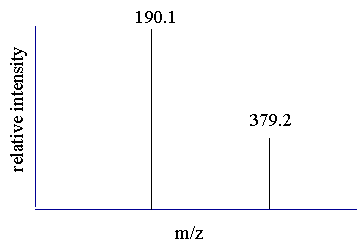
With the multiple charging phenomena displayed in electrospray spectra how does one calculate the mass of a compound? Doesn't the multiple charging effect just serve to confound the interpretation? How
does one determine if the ions are even related? |
 |
|||
| - | ||||
| Determining the charge state of the peaks. | ||||
| Before the mass of the compound is determined from the peaks in the spectrum a mathematical relationship must be established. One may assume that the peaks observed in a spectrum are related and then proceed to derive the relationship. The first assumption is that the two adjacent peaks are from the same compound and differ by only one charge. Before we can calculate the mass of the compound we must determine the charge state of the two peaks. | ||||
| Step 1 Understand that the peak value observed in the spectrum equals mass divided by charge and that the mass is the mass of the molecule plus any adducts (protons) | ||||
|
190.1 = mass/charge and 379.2 = mass/charge |
||||
|
these two equations can be rewritten |
||||
|
mass = 190.1 (charge) and mass = 379.2 (charge) |
||||
| Step 2 Assume that the two peaks are related, (charged variants of the same compound), and assume that they differ by a single charge. Since we have assumed that the unprotonated mass for these two peaks are equal we can set these two equations equal to each other and solve for the charge state of the peaks. | ||||
| If the two peaks are related and differ by one proton then more specifically we can write | ||||
|
m+1=190.1(z+1) and m = 379.2(z) |
||||
|
and |
||||
|
m = [190.1(z + 1)] - 1 and m = 379.2(z) |
||||
|
since "m" is assumed to be the same for both peaks we can set the two equations equal to each other and solve for "z" |
||||
| 190.1z + 189.1 = 379.2z | ||||
|
"z" equals |
||||
|
z = 1 |
||||
| This tells us that apparently the two peaks in the spectrum are mathematically related and that the charge state of the 379.2 peak is +1 and the charge state for the 190.1 peak is therefore +2. | ||||
| Step 4 Use the determined charged states to calculate the mass of the compound. One can think of the different peaks in the spectrum as separate mass measurements of the same compound. You can then average the two answers to get the final mass. See next page. | ||||
|
|
||||
 |

 |
|||
|
|
||||
|
home
| disclaimer |
||||