|
|
|
|
"Isotopes" Slide 2 |
|
|
. |
|
|
.Nomenclature
|
|
| . | |
|
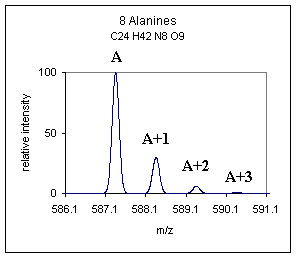
Molecules are made up of chemical elements and elements have naturally occurring isotopes. See the atomic mass table on this web site for some examples of naturally occurring isotopes and also take note of the fact that the isotopes all have unique abundances. For example the poly-alanine peptide that we have pictured below is rich in carbon, hydrogen, nitrogen and oxygen. Notice in the atomic mass table that the 13C isotope is the biggest contributor to the second peak in the spectrum below, next at about one third the contribution per mole is nitrogen. The theoretical isotopic distribution can be calculated using these masses and abundances. So the next time you see a spectrum like this you can say, "13C is the major contributor to that second peak", some call the second peak the 13C peak even though it is not made up entirely of 13C, ( 13C - pronounced C-thirteen, or carbon thirteen peak). |
|
|
|
|
|
. |
|
|
. |
|
| We will
follow the nomenclature for the monoisotopic and isotopic peaks as
described in Fred W.
McLafferty's book "Interpretation
of Mass Spectra." An example is shown in the figure
above, the monoisotopic peak is labeled "A" and the subsequent
peaks are "A+1", "A+2" etc. We highly recommend
McLafferty's book. The book covers interpretation of electron impact fragmentation
spectra but the basic mass spectrometry concepts are mode-less/timeless, in addition
it also contains a very useful mass spectrometry glossary.
The "A" peak is known as the "monoisotopic peak." Monoisotopic mass can be measured if the mass spectrometer used has sufficient resolving power to resolve the isotopes. Often on low resolution quadrupole instruments at higher mass (+2000 amu) the isotopic peaks cannot be resolved and the peaks will appear merged. "Exact mass" or accurate mass is the mass of the monoisotopic peak measured accurately to within a few millimass units (mmu). Exact mass can be used to determine the elemental composition of an unknown compound, but not to get ahead of ourselves, this subject will be discussed later. |
|
Next Slide
 |
|
| - | |
|
.(slide jumper) Contents Glossary Comp. 1 2 3 4 5 6 7 8 - |
|
|
home
| disclaimer |
|