|
|
|
|
"Isotopes" |
|
|
Slide 4 |
|
|
Exact Mass Measurement and Elemental Composition Determination
|
|
| . | |
| . | |
|
In determining the elemental composition for an unknown compound from an exact mass measurement it helps to know as much as possible about the molecule being studied. Even if the molecule is a true unknown it is never truly unknown. We can easily ask, "Is it a protein?", "Is it a small molecule?", "If it is a small molecule, what are the functional groups?" Use UV, IR, NMR, chemical modification, enzyme digest, or N-terminal protein sequencing to try and answer a few of these questions. A little bit of knowledge about the molecule can help you restrict the number of returns obtained from an elemental calculator. Determining elemental composition from an exact mass measurement for a molecule which is essentially unknown is very difficult. In this case for the purpose of elemental composition determination exact mass measurements are performed on molecules below 500 amu with a required mass accuracy at or better than 5 mmu. Higher mass accuracy is always helpful. With increasing mass the number of elemental combinations increases exponentially. One way to combat the large number of possibilities is to reduce the size of the unknown portion of the molecule. As mentioned above if the unknown is a protein, an enzyme digest may help reduce the size of the unknown portion of the compound, also if functional groups can be identified these masses can be subtracted from the measured mass to help reduce the number of returns. It is difficult to determine the elemental composition of larger entirely unknown molecules. This is not to say that exact mass of larger molecules is useless. This information can be used in conjunction with other analytical analyses and in this case it becomes a valuable tool. Exact mass is especially useful when a large part of the molecule is known and a minor unknown change needs to be characterized. Exact mass traditionally has been obtained by FAB magnetic-sector instruments and more recently by FTMS, MALDI TOF and ESI time of flight type instruments. Some scientists attempt to push their quadrupole mass spectrometers to obtain exact mass measurements. We have heard multiple reports of scientists using their quradrupole instruments to obtain exact mass, and while we believe their reports, in our experience we have been unwilling to claim better than 100 mmu accuracy for several of the most popular triple quadrupole instruments that we have worked with. As stated previously a mass spectrometer can never be too accurate or possess too much resolving power. As a side note, some higher end MALDI instruments are capable of routinely delivering mass accuracy for peptides to 40 mmu without an internal standard and to 5-10 mmu with an internal standard. In our experience the better MALDI instruments have beat the standard quadrupole or triple quadrupole instrument for consistent mass accuracy for peptide mass measurement, especially for peptides below 2000 amu.
|
|
| Procedure: | |
| . | |
| . | |
| . | |
|
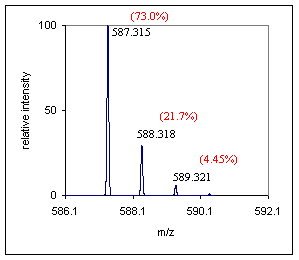
Step 1) Obtain an exact mass for the monoisotopic peak, gather elemental information from the A+1 and A+2 peaks. Look at the A+1 peak to estimate the number of carbon atoms, see the spectrum below. To estimate the number of carbons in this compound, divide 21.7 by 1.1. The estimate for the number a carbons in this compound is 19.7. Since the abundance of the A+1 peak is a measured quantity it might be wise to allow a range in this estimate of the carbon content of ~20-30%, depending on the mass spectrometers ability. If your instrument is capable of a more stringent measurement of the A+1 peak abundance this will help out enormously. By inspecting the spectrum below no unusual "A+2" type elements were identified, the peak looks to be at the appropriate abundance for a compound of this mass. |
|
|
|
|
|
(Spectra in this tutorial were generated using the IsoPro program, v.3.01) |
|
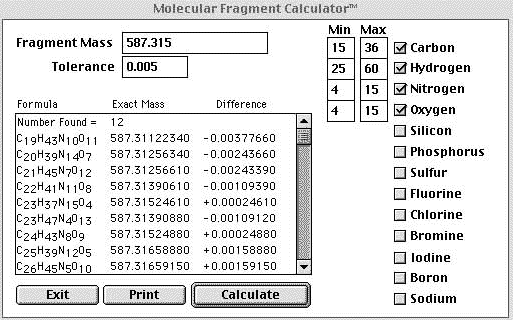
| Step 2) Use the
exact mass information to plug into a an elemental
calculator.
Rough estimates of the elemental ranges were made to cut down on the number of returns. The measured exact mass was entered into the calculator along with a tolerance of 5 mmu. |
|
|
The above figure is a screen shot of the program MFcalc™ made freely available by James E. Deline |
|
|
. |
|
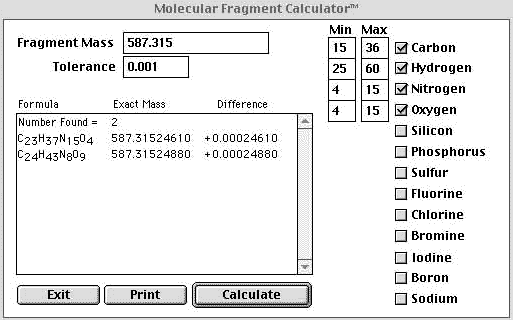
| In the example above liberal constraints were placed on the calculation and an unacceptable number of returns were obtained. Since obvious "A+2" elements other than oxygen were not observed they could be excluded from the search which helped us out a little. If we were confident that our mass accuracy was 1mmu or better we could eliminate the majority of the returns. In the example below a 1 mmu tolerance was used and the elemental constraints remained the same. | |
|
|
|
| If we had an instrument that could reliably deliver mass accuracy to1 mmu or better we could cut out a large number of the possible combinations. Above we have constrained our mass accuracy to 1 mmu and the returns were cut down from 12 to 2. At this point we can apply the nitrogen rule, the reader is referred to Fred McLafferty's book, Interpretation of Mass Spectra. The nitrogen rule states that if the molecular ion is odd then the compound will have an odd number of nitrogens. Since we are dealing with protonated species in electrospray and not molecular ions we can adapt the rule and restate it as, "If the protonated pseudo molecular ion gives an odd mass then the number of nitrogens is even." Since our mass is odd the number of nitrogens is even and we can eliminate the top return and therefore the protonated elemental composition of our unknown is C24H43N8O9. | |
| Step 3) As a last resort, if more
than one return is obtained insert these formulas into a program that
will perform isotope simulations then compare the isotope percentage
obtained from the simulation to the measured abundance of the
peaks. In addition rings and double bonds can be calculated for a given
composition which may help eliminate some choices, again the reader
is referred to Fred McLafferty's book, Interpretation of Mass
Spectra
This example illustrates the difficulty of elemental composition determination of a large unknown. It always pays to do your analytical home work and learn as much about your unknown molecule as possible. No one technique will lead you to the answer and each result becomes more relevant when placed in the context of other data. |
|
|
Suggestions |
|
|
. |
|
| The result you get from an exact mass measurement must be reliable, or it is not worth performing the experiment. One must be entirely confident of the instrument one is using and be aware of it's limitations. One good way to know your instrument is to measure test compounds of known composition. Another caution is not to limit any characterization just to an exact mass measurement. We advise you to learn as much about your molecule as possible. Here are just a few suggestions. Much can be learned from chemical modification of the unknown followed by mass measurement. If you believe that the unknown molecule contains the amino acid residue cysteine try performing an alkylation. If you believe that the unknown contains primary amines try an acetylation. To discover unknown carboxyl groups (COOH) try a methylation. For example if one is able to identify two carboxyl groups by the addition of two methyl groups then 2(COOH), 89.9953 amu can be subtracted from the overall exact mass result. The new smaller number will return fewer hits from an elemental calculator. If enough material is available, usually mg amounts, NMR along with exact mass is normally enough to give the elemental composition and in many cases solve the structure of the unknown. Another recommendation is to validate the results from any elemental calculator. The best calculators will allow user modification of the isotopic masses to bring them into agreement with the most current IUPAC values. | |
Next
 |
|
| - | |
|
.(slide jumper) Index Glossary Comp. 1 2 3 4 5 6 7 8 - |
|
|
home
| disclaimer
|
|