Protein ID With MS/MS Data
Step 1. Step one usually begins with
placing an enzyme specificity constraint. Some programs pre-index the sequence
database based on the enzyme specificity. Indexing makes the search go
much faster. For example theoretical tryptic peptides can be generated, and mass lists
can be pre-made from a protein database. The downside is a separate database needs to be indexed for each
enzyme, or each time a potential modification is changed.
-
Step 2. Step two involves matching the
parent mass of the intact peptide to the peptides in the database. Generally
the narrower you can set the parent mass constraint the faster the search
will go because fewer peptides will need to be correlated in the next step. One needs to be certain
that the accuracy of the mass spectrometer is not exceeded when setting the
parent mass window in the search. In this step a short
list of peptides are selected for the fragment mass correlation in the next step.
-
Step 3. Step three takes the list of
peptides identified by parent mass in step two and compares the theoretical fragment
masses of these peptides to the experimentally derived fragment spectrum.
-
Step 4. Peptide Ranking. Hits are ranked by
how many of the fragment masses match the theoretical fragment masses in the
sequence database.
Step 5. If more than one peptide is
searched all of the peptides found are correlated to their prospective
proteins. The protein with the greatest number of well correlated
peptides is usually the most significant hit.
-
Step 6. Some programs go a bit further using probability to
back-up the proposed match. Most users like to have a real probability
number, this gives them some comfort in the designation. Probability
takes the onus off of the user. While probability comforts us, we find
for the most important individual protein hit we still go in and manually
validate the spectral match.
Simple MS/MS Protein ID Exercise:
Use the MS/MS spectrum in Figure1 below to search
a sequence database. For this example we will be using the web version
of the database searching program
Figure 1
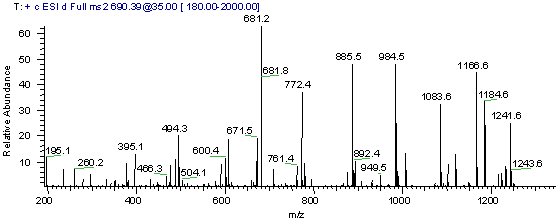
Exercise Procedure:
You can use the dta file
that we have already created for you, right
click on this dta link,
and save this file to your computer, choose save target as. When
saving the file to your computer, during the saving dialog you may
need to select "all files" as the file type and you may also need
to add the suffix .dta to
the "msms" name, in order for your computer to get the file type
correct. A dta file
is a mass / intensity pair list that is a representation of the original
MS/MS spectrum, the dta file is a Thermo Finnigan file format. The dta file
is very small, a few KB, once you have it downloaded, if you want, you can
open it with notepad to inspect the simple file format. The file should
have the name msms.dta
Steps to follow on the Mascot submission page:
1.) Follow this
link
to the Mascot web portal.
2.) On the welcome page click on the "Mascot"
link.
3.) On the search page click on the MS/MS "Ion
Search" link, and the search dialog will come
up.
4.) We will make this easy
A.)
You will need to enter a name and an e-mail address. You can spoof these if
you really want to.
B.) For
the database pick SwissProt
C.)
For the enzyme choose Trypsin
D.) Look for the
data format
field, and change the file format to Sequest
(.DTA)
E.) Under the instrument field you can choose
ESI Trap
F.) Go to the data file field and browse to your data file called
msms.dta, and pick it to
upload.
G.) Click on the
Start Search
button.
.
The search should only take a minute to complete. Do not browse away, and the result will come up automatically. If you put in your correct e-mail address Mascot would send you a link to your results via e-mail. When the results come up, click around and explore the different links associated with your result.
Inspect the results page:
Conclusion:
Mass spectrometry based protein identification is too easy right? So far we have used three MS sequence database searching techniques to identify a single protein. Continue on to the final tutorial to learn how to identify many proteins in a somewhat typical proteomics experiment.
References:
-
Jimmy K. Eng, Ashley L. McCormack and John R. Yates, III An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database JASMS, Volume 5, Issue 11,
November 1994 ,Pages 976-989 -
Yates JR 3rd, Eng JK, McCormack AL, Schieltz D. Method to correlate tandem mass spectra of modified peptides to amino acid sequences in the protein database.
Anal Chem. 1995 Apr 15;67(8):1426-36. PMID: 7741214 -
Yates JR 3rd, Eng JK, McCormack AL. Mining genomes: correlating tandem mass spectra of modified and unmodified peptides to sequences in nucleotide databases.
Anal Chem. 1995 Sep 15;67(18):3202-10. PMID: 8686885 -
Lim H, Eng J, Yates JR 3rd, Tollaksen SL, Giometti CS, Holden JF, Adams MW, Reich CI, Olsen GJ, Hays LG. Identification of 2D-gel proteins: a comparison of MALDI/TOF peptide mass mapping to mu LC-ESI tandem mass spectrometry. J Am Soc Mass Spectrom. 2003 Sep;14(9):957-70. PMID: 12954164


Copyright � 2004-2016 IonSource All rights reserved.
Last updated: Tuesday, January 19, 2016 02:48:20 PM
