Return to IonSource HomepageApplications of Capillary HPLCClick on the thumb nails in this column for a larger view |
|
|
1) Fast Unknown or Variant Characterization.
Fast Unknown or Variant Characterization: The traditional approach, to answer an analytical question usually involves 3nth steps: 1) Preparative isolation of the unknown, 2) Subjecting the unknown to a battery of analytical tests. 3) Re-isolation of the unknown after the initial isolate is used up. The preparative isolation step in a conventional characterization can take two to four weeks of precious lab time. In the conventional characterization, step two is often short lived as the small amount of the unknown is too soon consumed. The second step is followed by endless cycles of separation and collection of the minute precious unknown. The obvious but often overlooked solution is to isolate a large amount of the unknown, but this is not always possible due to the unknowns rarity. The natural question arises, how much unknown is needed to answer the analytical query, "What the heck is this stuff?" Since the substance is often unknown or only partially know this question is unanswerable. The time sink in this type of characterization is the vicious cycle of isolation, characterization, and the need to re-isolate. More often than not the substance is difficult to obtain or may not even be re-obtainable. About this time the director comes in to the lab to ask, "What is the progress on the project? What shall we tell the project team?" The most common answer is, "We don't know what it is and we will need to isolate more." This tormenting cycle of isolation and attempts at characterization and re-isolation is a common theme in many characterizations. Time and money are wasted and scientific races are often lost and the agony of not meeting ones corporate or academic goals are the result of the traditional characterization process. How does one answer the question, "What is this stuff?" The answer is quite simple, a researcher only needs to ask the right questions in the traditional investigation. The difficult part is knowing which questions to ask. For a total unknown this is an impossible criteria for success. The key is that the researcher needs to approach this problem like a player in a game of twenty questions. The first question may be "Is this a protein?" which maybe answered by a proteolytic digest and a chromatographic analysis. The second question could be, "What is the amino acid sequence delivered by an MS/MS fragmentation during an LC/MS run of the proteolytic fragments?" The truth is that any characterization can be accomplished in a reasonably short time if one is able to ask an unlimited number of questions, it is exactly like playing the twenty questions game. The problem that the researcher is faced with is that the minute amount of the unknown preparatively isolated is too soon consumed using conventional (macro) techniques and the substance runs out before the questions do. Capillary Chromatography can greatly help you on the road to characterization success. A typical amount for analysis on a narrow bore column (2.1mm ID) is 200-1000 pmol, for a 50 kDa protein this is 10 to 50 µg. For an unknown isolated from a polyacrylimide gel 10 to 50 µg is an impossible amount, even for a process variant 50 µg is significant. On a narrow bore column one may be able to answer one question with 1000 pmol. Remember for that 1000 pmol amount this is often a one shot analysis, and even if you think that you can use 200 pmol per analysis it is often the case that the refining of that one chromatographic question will take five injections. With capillary chromatography a chromatographic or LC/MS query is easily accomplished with just 5 pmol. If one has isolated 1000 pmol of a process variant, 200 questions can be posed chromatographically or by LC/MS. With 200 questions in your pocket one can begin to probe the mysterious entity chemically, enzymatically and chromatographically. Even the most difficult characterization can be accomplish in this luxurious environment. The term micro-preparative at first glance appears to be an oxymoron, but in actuality micro-preparative is a technique that is one of the major strengths of capillary chromatography. Capillary mobile phase flow rates are typically 3 to 8 µL per minute and peaks widths typically range from 0.5 to 1.5 minutes. The low flow rate and high peak concentrations allow for collection of analytes typically in 5 to 10 µL. The low volume and high peak concentrations help to defeat absorptive losses. Absorptive losses to large surface areas is one of the major hurdles in micro characterization. In a micro-preparative fashion, amounts as large as 1 nmol can be loaded onto a 320 micron ID capillary column for concentration and then eluted in 5 to 10 µLs. A similar purification on a standard column may result in a collection volume ranging from 0.2 mL to 1 mL. A significant portion of 1 nmol of a sticky protein or small molecule may be lost in the latter purification. In addition the 1 ml volume may require an additional manipulation to ready the unknown for further studies. After a micro preparative collection tiny aliquots, (0.5 µL), can be manipulated without need for concentration or lyophilization and can be taken directly to the next analysis either through dilution or directly, saving time and material. As a Tool for Proteome Investigations: Proteome studies have gained prominence recently as we enter the "post genome era". The identification of silver stained protein spots from 2D gels using mass spectrometry has been the method of choice throughout the '90s and most likely will continue to be a popular method for some time. In addition the importance in the characterization of post-translational modifications has also be recognized. With a 2D gel one can separate as many as 2000 proteins in a single run. The one downside to the 2D separation technique is the ultimate capacity of the gel. Only high µg amounts of material can be loaded onto the gel. Individual spots from 2D gels maybe contain between 50 fmol to 2 pmols of protein. 50 fmol of protein embedded in gel presents the the proteome investigator with a real challenge. Many have turned to MALDI-TOF mass spectrometry to make these identifications. Others have realized that any co-migration of proteins in a spot will confound protein identification when using MALDI-TOF peptide mass fingerprinting. In an attempt to get around this limitation MALDI-TOF operators have used PSD (post source decay) and have identified proteins from peptide fragment spectra. PSD is a technique that is not easily automated. Some of the best labs have turned to capillary chromatography and nano-electrospray where peptides are separated chromatographically and identified in an automated fashion using ion trap or triple quadrupole type mass spectrometers. Very low fmol amounts are routinely identified on 100 µm ID reverse phase capillary columns. Most researchers perform in-gel trypsin digestion and LC/MS, MALDI or N-terminal sequencing analysis to identify proteins from 2D gels. As an alternative to in-gel digestion an on-blot digestion can be performed after an electroblot procedure. On-blot digestion is often performed if special visualization (western analysis) are required, also if the gel is to be shipped to a collaborator electroblots are often more convenient.
In the middle days of electrospray (late '80s to the early '90s) capillary HPLC enjoyed a burst of activity since most LC/MS systems in that period functioned optimally between 2 and 15 µL/min. This was a flow rate that suited both capillary HPLC and mass spectrometers very well. Since mass spectrometers are concentration-dependant detectors the increased peak concentrations gave mass spectrometers more than just a suitable flow rate for LC/MS analysis. Over the past ten years capillary HPLC mass spectrometry has grown to be the technique of choice for characterizing samples at low abundance.
|
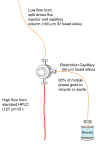 Figure 1 |
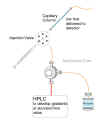 Figure 2 |
|
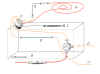
Figure 3 |
|
|
. |
|
|
TOC Introduction Glossary Simple System Automation UV Detection Plumbing The Truth About Splitting Applications Order Components References |
 |
| - | |
|
home
| disclaimer |
|